Ultrasound for vascular access in newborn pigs with Color Doppler
February 5, 2021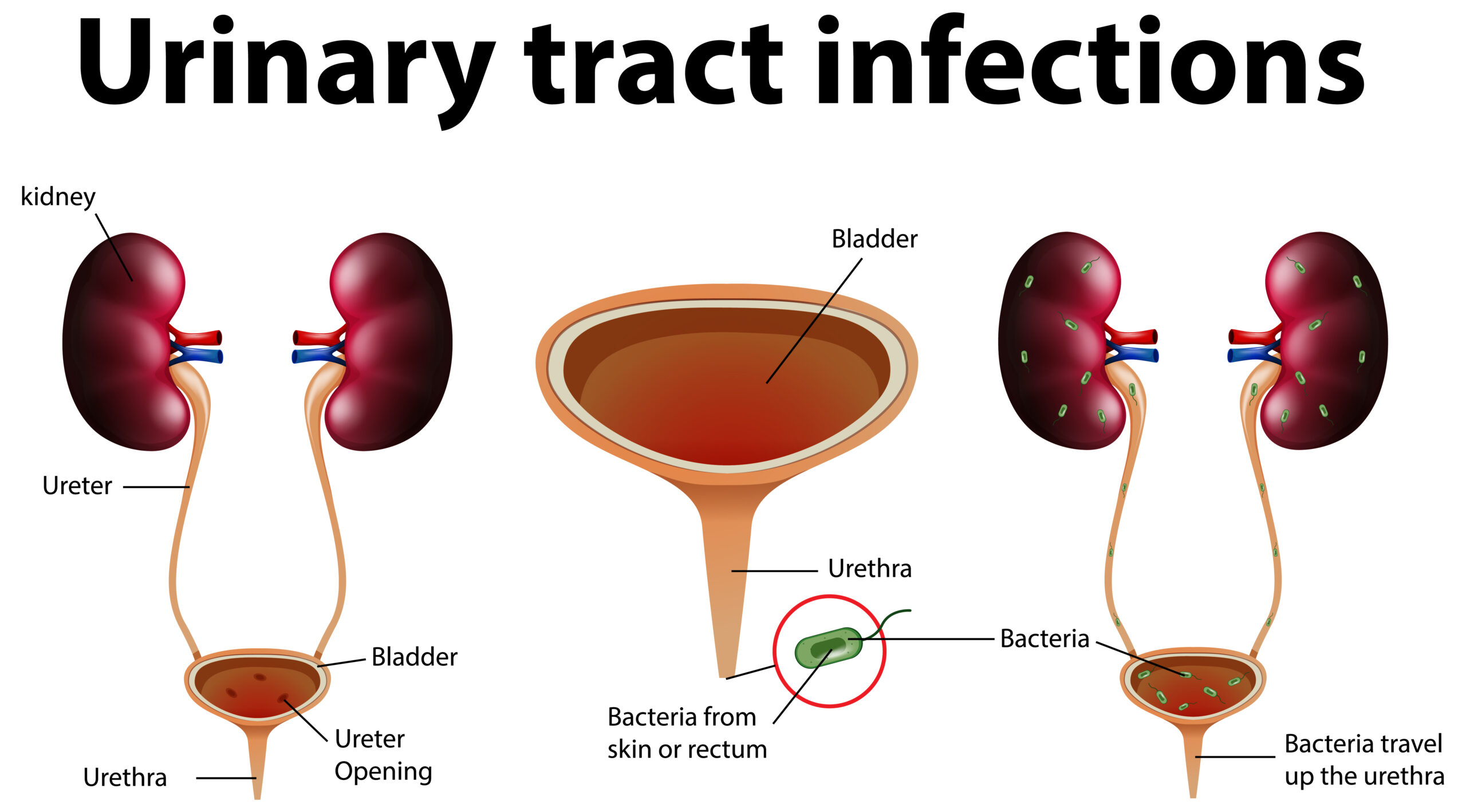
Urology: Urologic Surgery and Trauma Experience
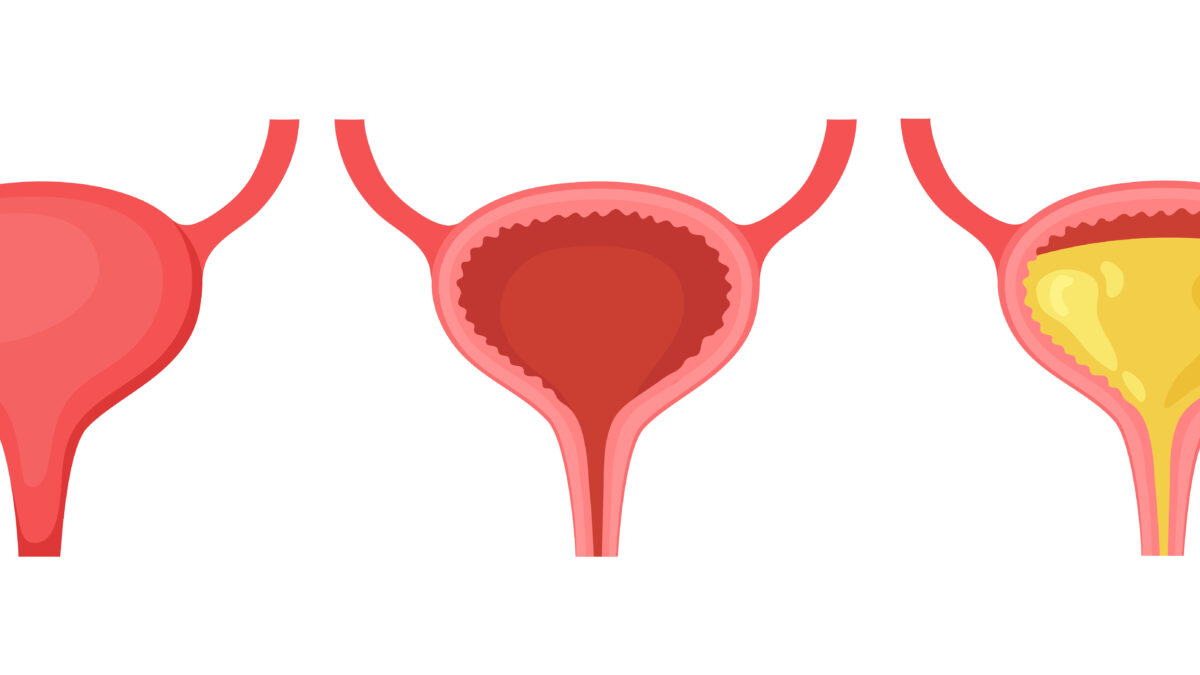
March 24, 2021Post-void residual volume (PVR) is the quantity of urine stored in the bladder following a voluntary void which serves as a testing aid.
This helps evaluate several disease procedures: neurogenic bladder, cauda equina syndrome, urinary exit obstruction, mechanical obstruction, medication-induced urinary retention, postoperative retention of the urine, and urinary tract.
In particular, urinary retention can be asymptomatic or induce urinary frequency, a feeling of inadequate emptying, and urge or overflow incontinence.
It can cause distention and pain in the abdomen. When retention progresses steadily, there may be no discomfort. Long-term retention is predisposed to UTI and can increase the pressure of the bladder, causing obstructive uropathy.
The post-void residual examination is usually done using ultrasound, a bladder scanner, or a urinary catheter (a direct measurement of urine volume).
There are several benefits of portable ultrasound bladder, including the fact that ultrasound bladder screening is more practical for the patient, brings a lower risk of infection since it is non-invasive, and is relatively simple and easy to use, saving health workers/ nurses time.
The Wireless 4D Bladder Ultrasound Scanner B-4D FDA, for example, is ideal for testing bladder drainage. It has good bladder wall recognition technology (high recognition rate with air for the bladder wall) and better probe accuracy.
In brief, Post Void Bladder Ultrasound screening is a method for patients with urinary retention complications to assess the volume of urine left in the bladder after urination (or voiding) has occurred (inability to clear the bladder completely).
Reference: Bladder Post Void Residual Volume,
Disclaimer: Although the information we provide is used by different doctors and medical staff to perform their procedures and clinical applications, the information contained in this article is for consideration only. SONOSIF is not responsible neither for the misuse of the device nor for the wrong or random generalizability of the device in all clinical applications or procedures mentioned in our articles. Users must have the proper training and skills to perform the procedure with each ultrasound scanner device.
The products mentioned in this article are only for sale to medical staff (doctors, nurses, certified practitioners, etc.) or to private users assisted by or under the supervision of a medical professional.